Affimed Therapeutics B.V. - AFMD
Seite 34 von 68 Neuester Beitrag: 21.05.25 14:43 | ||||
| Eröffnet am: | 16.09.14 15:03 | von: NikGol | Anzahl Beiträge: | 2.697 |
| Neuester Beitrag: | 21.05.25 14:43 | von: Highländer49 | Leser gesamt: | 677.447 |
| Forum: | Hot-Stocks | Leser heute: | 409 | |
| Bewertet mit: | ||||
| Seite: < 1 | ... | 31 | 32 | 33 | | 35 | 36 | 37 | ... 68 > | ||||
weitere Korrektur nach oben brauchen, wenn erste Aktivität in den AFM24 Studien vermeldet werden kann - meine Meinung, KEINE Handelsempfehlung.
Dabei gibt es heute eine wesentliche Affimed Meldung: IND Zusage der FDA für die erste eigene Kombinationsstudie von AFM24 mit NK-Zellen von NKMax
Sehr gutes Timing vor Ostern und vor der AACR.
Meine Meinung - KEINE Handelsempfehlung.
Diesen Bereich 7,35/7,46/7,48 mal nachhaltig hinter sich lassen, das wär's
There were no observed events of cytokine release syndrome, neurotoxicity syndrome or graft-versus-host disease
Study is continuing enrollment of the second dose cohort
Company to host conference call/webcast on April 14 at 4:05 p.m. EDT
Heidelberg, Germany, April 9, 2021 – Affimed N.V. (Nasdaq: AFMD), a clinical-stage immuno-oncology company committed to giving patients back their innate ability to fight cancer, announced today positive initial clinical data from an investigator-sponsored study at The University of Texas MD Anderson Cancer Center evaluating cord blood-derived natural killer (cbNK) cells pre-complexed with Affimed’s innate cell engager (ICE®) AFM13 (CD16A/CD30).
This approach was developed in the laboratory of Katy Rezvani, M.D., Ph.D., Professor of Stem Cell Transplantation and Cellular Therapy at MD Anderson, who is presenting the data as part of the Major Symposia and Advances sessions at the virtual American Association for Cancer Research (AACR) Annual Meeting. The presentation is available for viewing by registered participants through June 21, 2021. Dr. Rezvani will take part in a live panel discussion as part of the presentation on April 13, 2021 at 1:30 p.m. EDT.
“We are encouraged by the initial safety and efficacy data from this groundbreaking first in-human study. The finding of an objective response rate of 100% amongst our first four patients enrolled is impressive,” said Andreas Harstrick, M.D., Chief Medical Officer of Affimed. “These initial results indicate AFM13 may have the potential to help NK cells target and destroy cancer cells. We plan to continue to develop and customize approaches that leverage the unique and differentiating features of our ICE® molecules in combination with adoptive NK cell transfer to provide options for treating a variety of hematologic and solid tumors.”
The open-label, non-randomized, single-center, dose-escalation trial is evaluating the pre-complexing of AFM13 with cbNK cells followed by three weekly infusions of AFM13 monotherapy in adult patients with recurrent/refractory CD30-positive lymphomas. The trial is led by Yago Nieto, M.D., Ph.D., Professor of Stem Cell Transplantation and Cellular Therapy at MD Anderson.
“There remains a high unmet need for effective treatments in relapsed/refractory (R/R) CD30+ lymphomas. We are encouraged by the data generated from the first patients treated with cbNK cells pre-complexed with AFM13,” said Dr. Rezvani. “The results suggest this combination is facilitating clinical responses with minimal toxicity, warranting further study as we continue to explore novel cell therapies for our patients.”
As of March 31, 2021, three patients have been dosed with two cycles of therapy in dose cohort 1 (1x106 AFM13-cbNK/kg) and one patient has received a single cycle of therapy in dose cohort 2 (1x107 AFM13-cbNK/kg). The study is currently enrolling patients in the second dose cohort of NK cells, and further updates are expected later in 2021. Results from the first cycle of the first dose cohort are being presented by Dr. Rezvani at AACR, and Affimed is supplementing the data with best responses as of March 31, 2021, as summarized below.
Patient number
cbNK Cell Dose
Patient
Cancer Type
Prior Treatment
CRS/ Neurotoxicity/ GVHD
Best Response
Cohort 1 – completed
#1
1x106 / kg
43-year-old-male
Hodgkin lymphoma
4 lines of therapy (ABVD, ICE, brentuximab vedotin, nivolumab + ruxolitinib)
None
Partial response
#2
1x106 / kg
31-year-old-male
Hodgkin lymphoma
werden am 14-ten (heute bekanntgemachten MD Anderson Daten zur Kombination von AFM13 mit Nabelschnur-NK Zellen) und 15-ten April (Jahresbericht 2020)
(Nur so zur Info...Nicht als Prophezeiung gemeint)

- einfach zu verstehende 100% CR / PR
- zahlreiche weitere Katalysatoren absehbar (AFM24 AACR & ASCO, Roche, Roivant, NKGen, Artiva IPO)
- Amphivena AACR Präsentation und nahende IPO
- zwei kommende Analystenkonferenzen mit aktuell schon zahlreichen Upgrades
z.B. Credit Suisse
".. AFMD's value would only be limited by the pace of new targets
and ICE therapies from the pipeline. We expect partner Genentech to evaluate AFM26
(BCMA) plus NK cells to compete with BCMA CAR-Ts in multiple myeloma, which is
upside to our estimates."
Dem kann ich nur zustimmen - trotzdem nur meine Meinung und keine Handelsempfehlung.
P.S. #839 die Kapitalmaßnahmen waren m.E. gut überlegt und sehr passend plaziert
nach unten kommen ....
Einer der besseren Artikel zu den Affimed-Daten von EndPts
Der Positiv Volumen Indikator OBV hat den Höchststand eingestellt und sollte er in den nächsten Wochen ein neues Hoch erreichen, wäre es für mich der nächste Fingerzeig, dass es weiter Richtung "blue sky" geht.
Schaut man nach dem seit 2020 startenden Aufwärtstrend, so ist ein Rücklauf auf Richy´s 7,5 Zone allerdings immer noch drin.
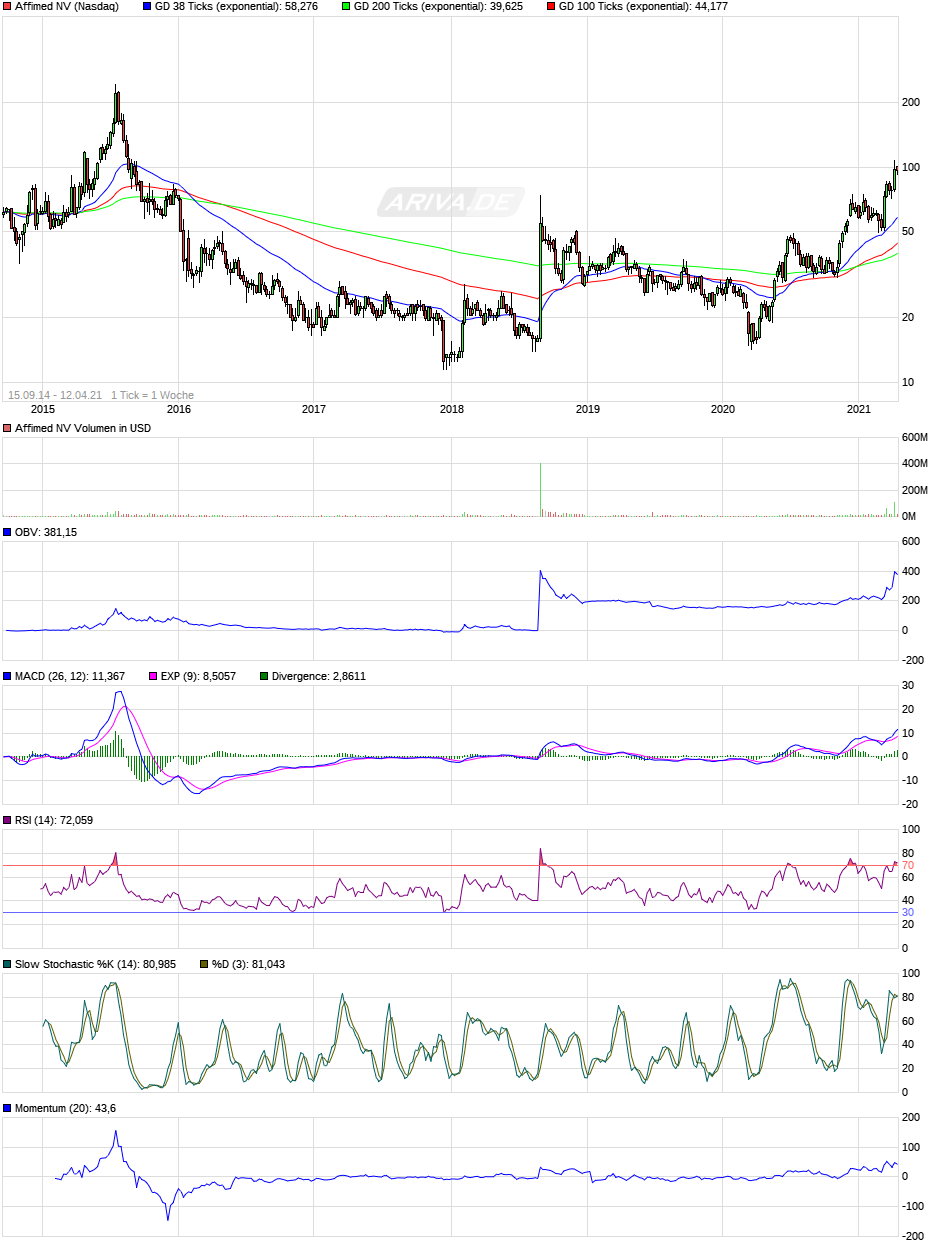
Bin gespannt, wie der Monatsschlusskurs wird.
eher von Vorteil ist. Die Überraschungskiste scheint noch groß und tief zu sein und Affimed am
Kapitalmarkt eher unterschätzt zu werden. Dies folgere ich zumindest aus den zuletzt geposteten
Kommentaren.
Schön wäre es, wenn Du die Vergleichsquote zukünftig noch nennen würdest.
Gruß rabautz
https://www.nasdaq.com/market-activity/stocks/afmd/short-interest
